The mechanism of how electrospray transfers charge from solution to analytes to form ions remains controversial. In 1968, Malcolm Dole first proposed the charged residue model. He hypothesized that the droplet retains the same charge during evaporation. At some point, the surface tension can no longer counter the repulsive forces from the charge on the droplet, causing the droplet to “explode” into many smaller progeny droplets. This Coulombic fission continues until only one analyte ion remains in the droplet. Finally, solvent evaporation leaves a gas-phase ion.
In 1976, Iribarne and Thomson proposed an alternative model called the ion evaporation mechanism. They suggested small droplets form via Coulombic fissions similar to the Dole model. However, the ion evaporation theory states that the electric field on the droplet surface is strong enough to energetically eject solvated ions from the surface directly into the gas phase.
It is possible both mechanisms cooperate: the charged residue model dominates for ions above 3000 Da, while ion evaporation dominates for lower ion mass ranges.
Reference:
http://R Cole, Some tenets pertaining to Electrospray ionization mass spectrometry, J of Mass Spec, 35, 763–772 (2000)
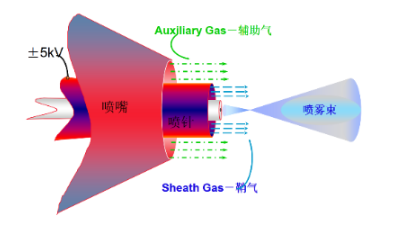
https://www.waters.com/nextgen/cn/zh/education/primers/the-mass-spectrometry-primer/common-ionization.html