In organic analysis, mass spectrometers are very commonly used instruments. Mass spectrometers consist of several major parts including the sample introduction system, ion source, mass analyzer, detector, and data system.
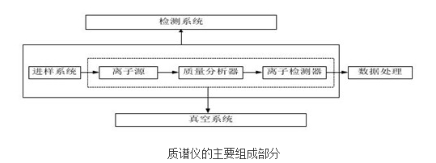
Mass spectrometers are typically classified based on their mass analyzer because the mass analyzer determines the resolution and capabilities of the instrument. Today we will discuss the ion source, which can be considered the “heart” of the mass spectrometer. Ionization of the sample is required for detection by mass spectrometry. The ion source generates ions from the analyte molecules and focuses them into an ion beam with defined energy and geometry. Due to the diversity of samples and analytical requirements, different ionization principles and methods exist to meet various needs. Therefore, in actual experiments, we need to select an appropriate ion source based on the sample properties and ionization principles.
The five most common ion sources are electron ionization (EI), chemical ionization (CI), electrospray ionization (ESI), atmospheric pressure chemical ionization (APCI), and matrix-assisted laser desorption ionization (MALDI). Our testing center is mainly equipped with EI, ESI, and APCI sources. So what are the ionization principles of our equipped sources and what types of samples are they suitable for?
Electron Ionization (EI):
EI sources are used in gas chromatography-mass spectrometry (GC-MS) and employ a "hard" ionization method. The EI source consists mainly of an ionization chamber, filament, ion focusing lenses, and a pair of magnets. The main working principle is that electrons emitted from the heated filament are focused by lenses and deflected by a magnetic field to pass through the ionization chamber and reach the collector. Sample molecules entering the ionization chamber are ionized by the 70 eV electrons. Ions with sufficient internal energy can fragment spontaneously upon colliding with neutral species like He to produce more fragment ions. All ions are focused and accelerated into an ion beam entering the mass analyzer.
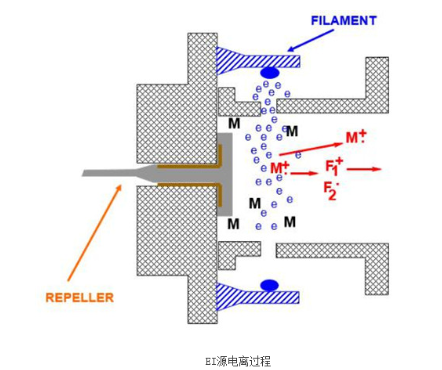
For most organics, EI produces both molecular ions and abundant fragment ions, facilitating structural interpretation. Standard mass spectral libraries utilize EI at 70 eV to ionize and fragment pure organic compounds to generate information-rich spectra. These standard spectra are compiled into libraries. Sample spectra obtained under the same conditions can be compared to library spectra for qualitative analysis.
However, for thermally labile samples, the molecular ion peak is weak or absent. Samples that cannot be vaporized or decompose upon heating also lack observable molecular ion peaks.
Applicable materials: volatile, thermally stable organics, typically boiling points below 500°C and molecular weights below 1000 Da.
Electrospray Ionization (ESI):
ESI is commonly used in liquid chromatography-mass spectrometry systems and causes minimal fragmentation, thus is a "soft" ionization method. The main working principle is: the sample solvent enters the electrospray probe through a capillary with applied high voltage. The high voltage charges the liquid surface, the surrounding heated nitrogen gas evaporates the solvent, and increasing Coulombic repulsion from solvent evaporation causes droplets to explode into individual ions entering the mass analyzer.
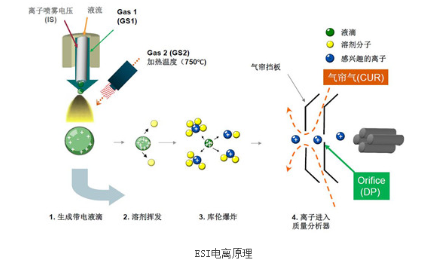
ESI generates mostly molecular ions due to its soft ionization, making it suitable for molecular weight confirmation. It does not decompose large, labile compounds during ionization. ESI is well-suited for highly polar, high molecular weight organics like pharmaceuticals, peptides, and carbohydrates. A key advantage is the generation of multiply charged ions, allowing high molecular weight compounds to fall within the mass range of common mass analyzers. For example, a 10,000 Da compound with 10 charges has an m/z of 1000, accessible to many mass spec systems.
However, ESI requires analytes to ionize from solution. Buffer type and concentration significantly influence sensitivity, so mobile phase selection is critical. Matrix suppression effects are also pronounced.
Atmospheric Pressure Chemical Ionization (APCI):
APCI lies between ESI and EI, and is mainly used in liquid chromatography-mass spectrometry. It generates quasi-molecular ions like [M+H]+ and [M-H]- with minimal fragmentation. The main working principle is: the sample flows through a heated nebulizer which assists evaporation. A corona needle continually discharges to ionize O2 or N2 molecules within the source. O2 or N2 ions transfer charges to solvent molecules which subsequently transfer charges to the analytes, generating sample ions entering the mass analyzer.
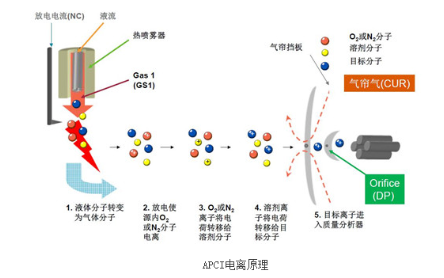
Atmospheric pressure chemical ionization is mainly used to analyze medium and low polarity small molecule compounds. Some analytes do not ionize efficiently under ESI due to structural and polarity limitations. In such cases, APCI can be used to boost ion yields and is considered complementary to ESI. APCI predominantly generates singly charged ions, so compounds analyzed are generally under 2000 Da molecular weight. Fragment ions are minimal; the mass spectra show mostly quasi-molecular ions.
APCI requires analytes to have some volatility for gas-phase ionization. Thermally labile compounds cannot be analyzed with this technique.
Reprinted from Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, China,Original link:https://kepu.nimte.ac.cn/view-14332.html