It has been 30 years since matrix-assisted laser desorption ionization, commonly known as MALDI, was first proposed by Hillenkamp and Karas in 1987. Since then, the application of this "soft ionization" technique in combination with time-of-flight mass spectrometry (MALDI-TOF MS) has successfully achieved the goal of providing rapid and highly reliable detection of biological macromolecules, as well as providing new analytical methods in the life sciences. Compared with other mass spectrometry techniques, MALDI -TOF is easy to operate and can be used by professionals who do not need to be trained in analytical chemistry. Especially in recent years, it has been increasingly favored in the field of clinical testing due to the development of applications such as genotyping analysis, biomarker identification, pathogen identification, and mass spectrometry imaging.
In recent years, the domestic R&D and production of MALDI-TOF MS instruments have started rapidly, and a number of researchers and enterprises have emerged, which has greatly promoted the localization of MALDI-TOF MS. MALDI-TOF MS will probably become the first mass spectrometer category in which the Chinese enterprises master the most advanced core technology and lead the development of the technology, which has a great impact on the research of China's analytical research of biomacromolecules, clinical molecular diagnostic applications.
I have been involved in the R&D and R&D management of a series of ESI and MALDI-based mass spectrometry products since 30 years ago, and I would like to take this opportunity of the 30th anniversary of the publication of the technology to make a brief review and outlook on the development of MALDI-TOF mass spectrometry technology and its future.
About the origin of MALDI
MALDI received the attention of the Nobel Prize Committee in 2002 at the same time as another "soft ionization" technology, electrospray ionization (ESI). Koichi Tanaka of Shimadzu Corporation, Japan, shared the 2002 Nobel Prize in Chemistry with John Fenn, the inventor of ESI, for "developing a method of soft desorption ionization for mass spectrometry of biological macromolecules" and for "developing a method of soft desorption ionization". "development of a soft desorption ionization method for mass spectrometry of biological macromolecules" shared the 2002 Nobel Prize in Chemistry. Although Koichi Tanaka's method used laser-based soft desorption ionization and thus won the Nobel Prize in Chemistry, he is often incorrectly credited as the inventor of MALDI. In fact, MALDI (matrix-assisted laser desorption ionization) was first proposed by scientists Hillenkamp and Karas at the University of Münster in Germany, and there are some fundamental differences between it and the method discovered by Tanaka. Although both methods use lasers, in MALDI the analyte is mixed into and surrounded by a matrix, and the matrix molecules absorb the laser energy and transfer some of it to the analyte (e.g., protein, nucleic acid molecules). Tanaka's method, on the other hand, uses a suspension of metal nanoparticles in glycerol, with the analyte sitting on the surface of the nanoparticles.
The matrix-assisted laser desorption developed by Hillenkamp and Karas was proven to be more efficient in ionization through practice, and became a widely adopted technique thereafter. However, Tanaka was the first to show that laser desorption ionization could be used to analyze and detect protein-based biomolecules, and reminded us that protein ionization alone was not enough, and that the analysis of biomolecules had to be accomplished by improving other parts of the instrument, especially the detector.
Strictly speaking, today's widely adopted MALDI-TOF mass spectrometry technology is actually a combination of two core technologies, i.e. matrix-assisted laser desorption ionization and time-of-flight ion separation. In addition to the combination of matrix-assisted laser desorption ionization and time-of-flight, it can also be connected with other ion separation means such as quadrupole and ion trap. However, pulsed laser desorption ionization certainly has many advantages over the coupling of pulsed ion extraction in time-of-flight mass spectrometry, leading to the emergence of MALDI-TOF as a mass spectrometry technique. The development history of this mass spectrometer is also the interdependence and mutual advancement of MALDI molecular ionization technology and TOF ion separation technology.
The design of pulse-triggered time-of-flight mass spectrometers appeared more than a decade before the advent of MALDI. Macfarlane et al. at Texas A&M University designed a Plasma Desorption Ionization (PDI) time-of-flight mass spectrometer by bombarding the surface of a sample with the radioactive element Californium-252, and used it to analyze organic molecules of higher polarity, which became the most successful mass spectrometer for protein macromolecule analysis at that time. used to analyze higher polarity organic biomolecules, it became the most successful mass spectrometry tool for the analysis of protein macromolecules and was considered the most promising at the time. the first commercially available product based on this principle was produced and marketed in 1984 by Bio-Ion Nordic AB, which was acquired by Applied Biosystems Inc. in 1989. However, this technology was short-lived and was soon replaced by the up-and-coming technology of laser desorption.
Laser Desorption has been studied for much longer, but had limited application in biomolecular analysis in the decades before the introduction of substrates that properly absorbed laser energy.Franz Hillenkamp, Michael Karas, and colleagues discovered in the mid-1980s that mixing alanine with tryptophan and irradiating it with a 266 nm laser pulse made it easier to convert alanine to tryptophan. pulses made it easier to ionize them, leading to the inference that tryptophan absorbs laser energy and helps ionize the non-absorbable alanine, thus giving the term matrix-assisted laser desorption ionization (MALDI). Melittin, a peptide with a molecular weight of up to 2843 Da, is also ionized when mixed with this "matrix". The breakthrough in laser desorption ionization of larger molecules came in 1987, when Koichi Tanaka and his colleagues at Shimadzu used an "ultrafine metal plus liquid matrix method" combining a 30 nm cobalt metal powder in glycerol with a 337 nm nitrogen laser for ionization. Using this combination of matrix and laser, Tanaka was able to ionize a biomolecule of carboxypeptidase-A protein with a molecular weight of 34,472 Da, demonstrating that the appropriate combination of laser wavelength and matrix can ionize the protein. Subsequently, Karas and Hillenkamp ionized 67 kDa albumin (Albumin) using a nicotinic acid matrix and a 266 nm laser.
Karas and Hillenkamp presented the spectra of β-galactosidase (molecular weight 116,900) obtained using a static electric field reflectance time-of-flight mass spectrometer coupled with MALDI ionization at the 1988 International Mass Spectrometry Conference in Bordeaux, the first time that a mass spectra of a singly-charged ion with a mass greater than 100,000 had been demonstrated, and marked the beginning of a new era of MALDI-TOF MS dawned a new era.
Karas and Hillenkamp presented the mass spectra of β-galactosidase (molecular weight 116,900) obtained using a static electric field reflectance time-of-flight mass spectrometer coupled with MALDI ionization at the 1988 International Mass Spectrometry Conference at Bordeaux
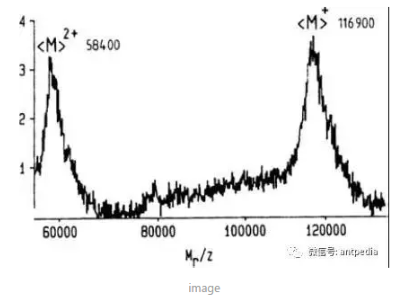
However, as can be seen from the peak widths of the mass spectra in the figure above, the protein volume fractionation obtained using static electric field reflectance time-of-flight mass spectrometry was quite low in the initial work. It was soon realized that this was mainly due to fragmentation of ions during flight.
Birth of the MALDI-TOF MS
The first practical MALDI-TOF mass spectrometer for macromolecular analysis was built by Beavis and Chait of Rockefeller University in the USA within a few months of the discovery of MALDI at Hillenkamp. It is a simple linear time-of-flight mass spectrometer with a monostatic accelerating electric field, drift tube, and detector. The instrument has an accelerating voltage of up to 30 kV and a flight length of 2 meters. A major advantage of the linear time-of-flight analyzer is that the dissociated fragmented ions arrive at the ion detector at nearly the same time as the stabilized ions during the flight, thereby attenuating the ion dispersion observed by Hillenkamp et al. in reflectance analyzers and improving mass resolving power.Beavis and Chait also carried out an extensive study of the substrates for the MALDI, which enabled a further improvements. Their development of cinnamic acid-derived matrices showed that any wavelength between the 260 nm and 360 nm UV bands could be used for laser desorption of proteins, allowing smaller and relatively inexpensive nitrogen lasers with a wavelength of 337 nm to be used in MALDI instruments as well, replacing the bulky and expensive Nd:YAG lasers, which were favored by commercial instrument developers in the early 1990s and are still widely used today. It was favored by commercial instrument development researchers in the early 1990s and is still widely used in commercial products today.
The first commercialized MALDI-TOF products appeared in the early 1990's. Vestec, founded by Dr. Marvin Vestal (acquired by PerSeptive Biosystems and then merged into Applied Biosystems Inc), and my company, Finnigan, developed and produced MALDI-TOF products in 1990, respectively. Finnigan's LaserMAT time-of-flight mass spectrometer was the first linear MALDI-TOF product to use a nitrogen laser, with a flight tube length of only 0.5 m. Vestec's time-of-flight mass spectrometer was the first to use a nitrogen laser, and Vestec's time-of-flight mass spectrometer was the first to use a nitrogen laser. The time-of-flight mass spectrometer produced by Vestec, on the other hand, utilizes a much longer flight tube and therefore performs better.
Compared to the rapid development and application promotion of the electrospray ionization mass spectrometry market, which appeared almost at the same time, the initial commercialization of MALDI-TOF products encountered some troubles. This is mainly due to the low performance of TOF instruments, especially the insufficient mass resolving ability of TOF, and the low mass measurement accuracy, such as the mass resolution of the above-mentioned Finnigan LaserMAT is only one or two hundred, which can not meet the demand of MALDI ionization for the detection of biological macromolecules. However, from another point of view, the great potential of MALDI ionization technology puts forward higher requirements for time-of-flight mass analyzers, which greatly stimulates the development of TOF instruments specially tailored and improved for this ionization technology.
Development of TOF technology
Time-of-flight (TOF) mass spectrometry was first proposed in the mid-1940s by W. E. Stephens of the University of Pennsylvania. Wiley and McClaren, then working at Bendix Aviation Corporation Research Laboratories in the United States, completed the design of the first practical time-of-flight (TOF) mass spectrometer in the mid-1950s, and systematically described the way to improve the mass resolving power, first proposed the pulse accelerated time The concept of focusing was first introduced with pulse acceleration, and the general conditions for achieving focusing were described. These conditions are also applicable to MALDI and other ionization techniques, which laid the theoretical foundation for the subsequent development of MALDI time-of-flight mass spectrometry. However, for a long time afterward, this technique was generally regarded as a luxury for basic research on ion properties, and was not widely used in analytical chemistry and in solving practical problems. It was not until the 1970s that the technique attracted renewed attention due to the emergence of pulsed ion sources such as plasma desorption ionization (PD), secondary ion mass spectrometry (SIMS), and especially matrix-assisted laser desorption ionization (MALDI).
A few key techniques for early high-resolution MALDI-TOF MS
Ideally, an ion source is supposed to produce a narrow and almost parallel ion beam, and the time of flight of ions of different mass sizes accelerated by an electric field to reach the detector is independent of the initial positions and velocities of the ions. Instead, during MALDI desorption ionization, the molecules to be detected are embedded in matrix crystals deposited on the surface of the sample target plate that serves as the electrode of the ion gas pedal. When a laser pulse irradiates the sample crystals, a plume of desorbed material including charged ions is generated. It is generally accepted that the ions fly out at an initial velocity distribution of hundred meters to kilometers per second. If two ions of the same size are accelerated in the flight tube at different initial velocities, they will arrive at the ion detector at different times, resulting in peak broadening. It is the difference in initial velocities between the ions that causes the linear MALDI-TOF resolution to decrease. Therefore one of the keys to improving the linear MALDI time-of-flight resolution is in refocusing the ion initial velocity distribution to compensate for the differences in ion initial velocities, which was specifically described by Wiley and McClaren in their 1953 publication, but has since been forgotten by most researchers.
After the advent of the MALDI ion source, in 1994 Lennon and Brown of Utah State University reported that the resolving power of MALDI-TOF could be significantly improved by employing precisely delayed extraction pulses after the ionizing laser pulse.Marvin Vestal immediately recognized and combined the already reported MALDI ion initial velocity distribution with the reported focusing correlations of linear and reflectance time-of-flight analyzers, Marvin Vestal immediately recognized and linked the reported MALDI ion initial velocity distribution to the focusing correlations of linear and reflectance time-of-flight analyzers, and spent nearly a year systematically developing theoretical models of the components in a TOF analyzer to optimize the performance of a TOF analyzer for MALDI ion sources. One of the most important efforts was the rediscovery and integration of Delayed Extraction, which was introduced into the design of the MALDI-TOF instrument. This technique involves varying the duration for which ions with different initial velocities are accelerated by the application of a delayed ion extraction electric field, so that eventually all ions reach the detector at the same time. This improvement increases the mass resolving power of the linear time-of-flight by more than a factor of 10.
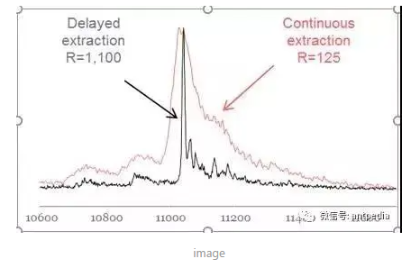
Comparison of Delayed Ion Extraction and Continuous Ion Extraction Mass Spectra
Another improvement to compensate for the difference in the initial kinetic energy of MALDI ion generation is through the inclusion of an ion reflector at the end of the linear time-of-flight tube. The concept of a reflector (Reflectron) time-of-flight, first proposed by Soviet scientist Mamyrin in 1973, consists of a series of uniformly spaced electrodes to which a reverse electric field in the direction of ion flight is applied. Ions of the same m/z in the flight tube will enter the reflector at different depths due to differences in kinetic energy and velocity. Ions with greater kinetic energy arrive and enter the reflector first, travel longer flight paths deeper into the reflective field than ions with less kinetic energy, and therefore arrive at the detector at the same time as the held-back ions. Building on Marvin's theoretical work, Applied Biosystems designed and developed the Voyager family of products, which provided the first high-resolution MALDI-TOF mass spectrometers for protein macromolecule analysis with mass resolving power in excess of 10,000 and mass accuracy up to 10 ppm. More than half of the commercially available MALDI-TOF instruments used in the market since the mid-1990s were based on Marvin's theoretical design.
Early MALDI-TOF instruments, while having many advantages, had one major limitation: they could only obtain the molecular weight of the analyte, but could not determine the structure. As a result, these instruments could not be used to determine protein sequences or post-translational modifications that are critical to protein chemists. In order to obtain protein and peptide structural information by MALDI-TOF, in the early 1990s, German scientists Kaufmann and Spengler utilized the so-called Post Source Decay (PSD) technique, in which substable decay ions are fragmented during flight in an electric field-free region and then separated by a reflector, thus obtaining information on the mass of the fragmented ions. Peptide sequencing was performed. This technique in a sense makes up for the inability of MALDI-TOF to obtain structural information of peptides, but after all, there is still a big gap between the precision of the molecular structure contained in PSD fragment ions and that of the usual MS/MS fragment ions. In order to make up for this deficiency, Marvin Vestal successfully constructed the design theory of MALDI-TOF-TOF instruments by refining the theory and extending it to tandem TOF mass spectrometry, and developed the 4700 Proteome Analyzer and later the upgraded version of the AB 4800 TOF-TOF Mass Spectrometer from Applied Biosystems. These instruments are still in use in most proteomics centers today. The MALDI-TOF MS and MS-MS systems have had a tremendous impact on many important areas of research, including proteomics, glycomics, cell signaling, structural biology, organelle imaging, and polymer science, and Marvin was honored with the American Society for Mass Spectrometry's highest award for contributions to MALDI-TOF and TOF-TOF in 2010. Distinguished Contribution Award.
In the early days of MALDI-TOF, it was mainly used for protein identification. One method of protein identification by mass spectrometry is to achieve protein identification by splitting the extracted proteome into peptides by endonuclease, and then comparing the degree of peptide mass match between the peptide peaks in the MALDI-TOF MS mass spectrometry map and the peptide mass generated from the protein database. This method is commonly known as Peptide Mass Fingerprinting (PMF). Another method to identify proteins is to bombard the generated peptides after protein endocytosis into ionic fragments in tandem mass spectrometry by MALDI TOF-TOF, and then search for matching peptide information in the protein peptide tandem mass spectrometry database to identify the proteins.
Compared to proteins and peptides, MALDI-TOF analysis of nucleic acids was relatively late in the game, and it was not until Becker's group in 1993 reported that 3-hydroxypyridinecarboxylic acid (3-HPA) could be used as a good substrate that a breakthrough occurred. One of these breakthroughs was the application of single nucleotide polymorphisms (SNPs) to the identification of gene loci. 1997 saw the first mass spectrometry method for SNP identification utilizing Single-base Primer Extension (SBPE) chemistry in combination with MALDI-TOF by Haff and Smirnov at Applied Biosystems. identification by mass spectrometry. The main advantage of this SNP assay is that up to dozens of SNP sites can be detected simultaneously in a single sample reaction system (e.g., the detection of breast cancer 1 gene shown in the figure).
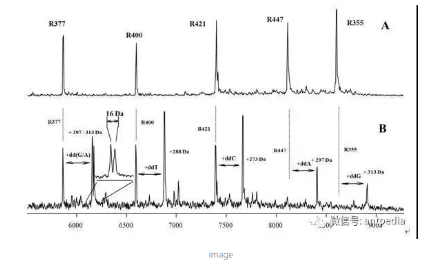
Mass Spectrometry for SNP Detection of Breast Cancer 1 Gene
Applied Biosystems has completed the development of a commercial product based on this method in 2000. However, due to business considerations, this product was not brought to the market. A commercial product based on single-base extension mass spectrometry really appeared on the market a few years later with the introduction of MassARRAY by Sequenom (now Agena). With the promotion of multi-locus SNP genotyping in clinical and precision medicine applications, the combination of single-base extension chemistry and MALDI-TOF assay has been increasingly emphasized.
Mass Spectrometry Imaging
After the emergence of MALDI-TOF technology, Richard Caprioli of Vanderbilt University in the United States realized that this molecular detection technology has the characteristic of two-dimensional scanning, and since the mid-1990s, he has begun the molecular imaging technology using laser scanning combined with mass spectrometry, exploring the development of a new method to determine the distribution of biological macromolecules in tissue sections, especially for the traditional immune system, which is the most effective way to determine the distribution of biological macromolecules. distribution, especially for samples that cannot be analyzed by conventional immunochemistry. The method they used was to spray frozen tissue sections with matrix droplets and scan the tissue samples with a MALDI-TOF instrument equipped with a fine laser focus. This technique, in addition to obtaining information about the molecules in the samples, also makes it possible to obtain the spatial distribution of biomolecules on complex surfaces in a chemically label-free state. This combination provides biological histology researchers with a chemical "microscope" that can be used for direct biomolecular characterization of tissue surfaces.
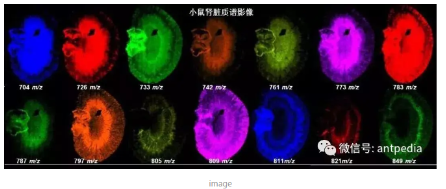
Mass Spectrometry Image of a Mouse Kidney
Richard Caprioli also received the Distinguished Contribution Award from the American Society for Mass Spectrometry in 2014 for his pioneering work in Imaging Mass Spectrometry.
Since its inception 30 years ago, MALDI has become a well-established technique for analyzing a wide range of non-volatile molecules, including proteins, peptides, oligonucleotides, lipids, glycans and other biomolecules. However, breakthroughs in routine clinical testing applications have only occurred in recent years for the identification of clinical pathogens, and in 2001, Ryzhov and Fenselau of the University of Maryland, USA, proposed the possibility of using MALDI-TOF for the identification of pathogenic microorganisms by mass spectrometry analysis of ribosomal proteins, a technique rich in molecular fingerprints in microbial cells. This mass spectrometry molecular fingerprinting technique was later validated to be more accurate and faster than various phenotypic and biochemical test methods typically used in microbiology-based laboratories. It also makes up for the lack of biochemical identification methods for the identification of difficult-to-identify and difficult-to-cultivate pathogens such as microaerobic bacteria, anaerobic bacteria, fungi, Mycobacterium tuberculosis, and viruses, and has been gradually adopted by clinical laboratories. Two foreign companies Bruker, bioMérieux launched a linear MALDI-TOF instrument based on the identification of clinical pathogenic microorganisms system has been approved by the U.S. Food and Drug Administration (FDA) and the domestic drug regulatory authorities for the identification of pathogens in clinical applications.
Limitations and improvements of MALDI
The technology has remained fundamentally unchanged since the advent of commercial products developed on the basis of the MALDI-TOF instrument design theory established by Vestal in the mid-1990s. However, we face a number of challenges to further advance this analytical method into the broader field of clinical testing and need to overcome many of the factors that have limited the widespread acceptance of this mass spectrometry instrument, such as the high cost of manufacturing and operational complexity of the instrument, its poor reliability, as well as its speed, full-spectrum sensitivity resolution, and the poor reproducibility of the mass spectra. In particular, these factors contribute to the general perception that MALDI-TOF is not a quantitative analytical tool, and further promotion in clinical analytical applications has suffered as a result.
To solve the problem of quantitative analysis by mass spectrometry from the application point of view, it is necessary to start from several aspects: sample pre-treatment, sampling method and quantitative analysis error of the instrument itself. Although Applied Biosystems developed the chemical reagents iCAT (Isotope-coded affinity tag) and iTRAQ (Isobaric tags for relative and absolute quantitation) at the beginning of this century, it has alleviated the problem of quantitative analysis to some extent. To a certain extent, the bottleneck of quantitative analysis has been alleviated, but the limitations of the instrument itself have not been solved. We need to look for the answer at the root of the problem, i.e., at the levels of ion generation, ion extraction and transport, and ion detection.
First of all, the crystalline layer of the sample and matrix molecules is unevenly distributed on the MALDI target plate, so a good quality mass spectrometry is obtained by searching for the "sweet spot" in the crystalline layer. Usually, the emission frequency of the nitrogen laser used in this type of instrument is limited to about 50Hz, so usually only a small portion (usually <1%) of the sample molecules are ionized and analyzed by the laser irradiation per unit of time, which makes the reproducibility of the ion intensities in the mass spectra of each superposition very unstable, with an error of up to 30% or more. The new generation of MALDI-TOF (e.g., QuanTOF of Rongzhi Biotech) adopts semiconductor lasers with 5000Hz or more, which, combined with fast 2D moving platform control and high-speed ion detection and data acquisition, can complete tens to hundreds of thousands of laser irradiations within the same time to ionize and analyze most of the samples on the target point, reducing the impact of the variation of sample quantity and uneven distribution.
Additional factors affecting the reproducibility of mass analysis include ion extraction within the MALDI ion source and the way the accelerating electric field is applied. In the previous generation MALDI-TOF design, these voltages were applied to the target plate, resulting in an uneven distribution of the electric field across the target plate, which caused ions spotted at different locations on the target plate to sense different electric fields, resulting in fluctuations in the time of flight of the ions. In the new generation of instrument design (e.g. QuanTOF), the patented technology of target plate grounding is adopted to eliminate the fluctuation of electric field at the edge of the target plate, further improving the reproducibility of mass spectrometry spectra. This improvement leads to better homogeneity of mass detection, which is particularly relevant for mass spectrometry imaging applications that require scanning in the spatial dimension.
Another improvement in the new generation of MALDI-TOFs is the detection performance in the full mass range.The inability of time-of-flight (TOF) mass spectrometry designs to focus different sized ions at the same time in the initial space and at different velocities was described by Wiley and McClaren in the 1950's.This, coupled with the asymmetry of the usual angle of incidence of the MALDI-TOF laser irradiation, makes the design of conventional MALDI-TOF instruments a challenge for the detection of different sized ions. -The innovative design and comprehensive improvement of the laser irradiation optical system, ion optical system, delayed ion extraction, ion focusing transport and mixed ion detector of QuanTOF have realized the simultaneous performance of resolution and sensitivity of mass spectrometry in the whole mass range, and improved the quantitative performance of mass spectrometry. The innovative design and comprehensive improvement of mass spectrometry have realized the simultaneous performance of resolution and sensitivity in the whole mass range, and improved the reproducibility of mass spectrometry quantitative analysis. The figure below demonstrates the ability to detect very high molecular weight proteins.
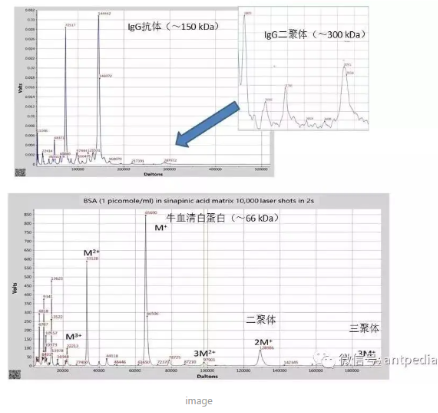
Mass Spectra of Very High Molecular Weight Proteins Detected by Next Generation MALDI-TOF
The improved quantitative performance of the new generation MALDI-TOF can be seen in the determination of hemoglobin glycation rate in whole blood samples. Shown in the figure below is an overlay of 72 mass spectra obtained from three hemoglobin samples with different glycation rates (6%, 9%, and 14%, respectively), each of which was repetitively spotted at 24 different points on the target plate. The reproducibility of the glycation peaks for the three different sets of ion intensities at different concentrations was more than 98%.
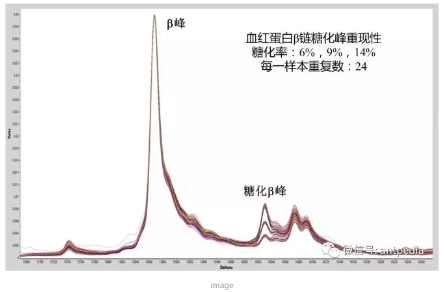
Next-generation MALDI-TOF mass spectra of hemoglobin samples with three different glycation rates
Full-spectrum quantitative MALDI-TOF will open the door to new applications.
Further development of the innovative technology is propelling MALDI-TOF into a brighter future as a standardized technique for individualized clinical testing of disease pathologies.The MALDI method can be used to analyze any body fluid containing the target analyte, including blood and blood products, breast milk, cerebrospinal fluid, lymphatic fluids, saliva, urine, gastric and digestive fluids, tears, stool, semen, prostate fluids, vaginal fluids, and other fluids. prostate fluid, vaginal fluid, amniotic fluid and interstitial fluids derived from tissues. In particular, the improved quantitative performance of the new generation of MALDI-TOF will allow for faster adoption into routine clinical testing, and in addition to pathogen identification, we will see this technology extended to: cancer typing directly from serum, tissue extracts, and other body fluids; tissue imaging; protein modification analysis; small molecule drug (in vivo) distribution; biomarker identification and validation; mass spectrometry immunoassays; peptide immunoassays; peptide immunoassays; and biomarker identification and validation; Mass spectrometry immunoassays; peptide quantification; clinical assays for diagnostic and therapeutically relevant biomarkers, etc.
Author: xiaosine
Link: https://www.jianshu.com/p/067f55ab22f8
Source: Jane's Book