Concept 1: Mass Spectrometry
An analytical method in which neutral molecules are first ionized, and then the mass-to-charge ratios and abundances of various ions are separated and recorded in turn to achieve the purpose of analysis.
Concept 2: Mass Spectrometry
A spectrum in which ions of different mass-to-charge ratios are separated by a mass analyzer and then detected and recorded is called a mass spectrum. It is called a mass spectrum for short.
The horizontal coordinate of the mass spectrum is the mass-to-charge ratio (m/z) and the vertical coordinate is the ion intensity;
Mass Spectrometry (Mass Spectrometry) that is, mass spectrometry, generally also referred to as mass spectrometry;
Mass Spectrometer (Mass Spectrometer): the use of a variety of mass ratio ions recorded in turn the intensity of the way to measure the mass spectrum of compounds;
Mass Spectrography: A mass spectrometer that records all ions simultaneously by means of a dry plate.
Concept Group 3: Mass Spectrometry Basics
Commonly used units of mass
Da=Dalton (Dalton)
mass unit, equal to a carbon atom (12C) one twelfth of the mass, about 1.66 × 10-24 grams; a gram is about 6 × 1023 Dalton.
amu=atomic mass unit ,atomic mass unit
1amu=1Da
A mass spectrometer instrument in which the atomic structure and its mass are recorded for all ions.
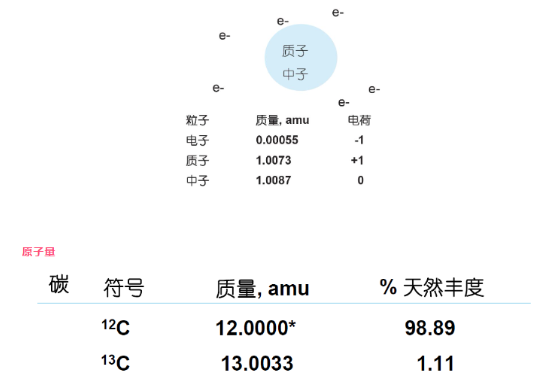
* :: International agreements give it an exact mass of 12
Atomic weight (C) = 0.9889(12.0000) + 0.0111(13.0033) = 12.011
The weight average of all isotopes of an element is called the atomic weight
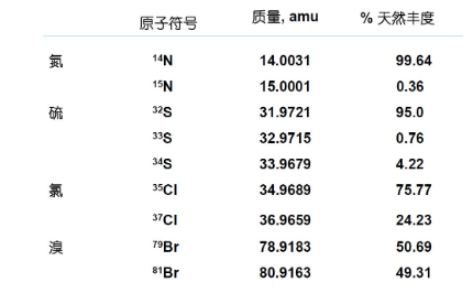
Isotopes and Isotopic Abundance
Isotopes i.e. atoms having the same atomic number but different mass numbers are called isotopes.
Isotope abundance, i.e. the percentage of atoms of an isotope in nature, is called the natural abundance of that isotope.
Isotope Representation
Mass number = Protons + Neutrons
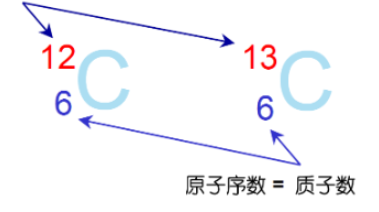
Have the same element symbol, with the mass number indicated in the upper left corner of the element symbol.
Concept 4: How to calculate mass number, molecular weight
Nominal mass number
Calculated using integers of elemental mass numbers, e.g. C=12, H=1, O=16
Single isotope mass number or exact mass number
Calculated using the exact mass number of the most abundant isotope.
Example: 12C=12, 1H=1.0078, 16O=15.9948
Average mass number or chemical mass number
Calculated by taking into account the atomic weight of the element for all natural isotope abundances
Example: C=12.001, H=1.00794, O=15.9994
The m/z value of a singly charged ion obtained by quadrupole mass spectrometry is the single isotope mass number, and it is recommended that mass spectral peaks be labeled to 1 decimal place.
Calculation of molecular weight
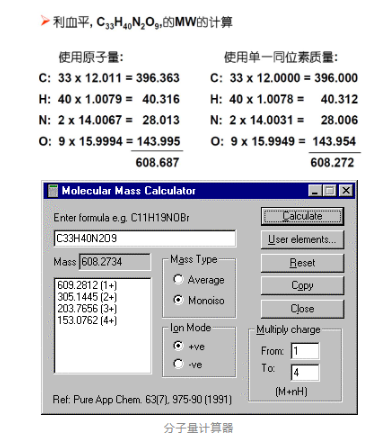
Concept 5: Nomenclature and Terminology for Mass Spectrograms
Mass charge ratio
The ratio of the mass of an ion (in relative atomic weight units) to the charge it carries (in electron charge units) is called the mass-to-charge ratio, abbreviated as m/z.
The mass-to-charge ratio is the horizontal coordinate of the mass spectrum.
The mass-to-charge ratio is the basis for the qualitative analysis of a mass spectrum.
Abundance of ions
The strength of the ion signal detected by the detector.
Relative abundance of ions
The intensity of the strongest peak in a specified mass-to-charge ratio range in a mass spectrometry, normalized to the intensity of the other ion peaks.
The relative abundance of ions is used as the vertical coordinate for all standard mass spectra.
The ion abundance of the peaks is related to the content of the substance.
Standard mass spectra are plotted with the relative abundance of ions as the vertical coordinate.
The ion abundance of the peaks is related to the content of the substance, and is therefore the basis for mass spectrometry quantification.
Base peak
In mass spectrometry, the ion peak with the greatest intensity within a specified mass-to-charge ratio is called the base peak. The relative abundance of the base peak is 100 %.
Back ground
The mass spectrometry signal, including chemical and electrical noise, detected under the same conditions as the analyzed sample when the sample is not fed.
Total ions current (TIC)
A plot of the sum of the intensities of all ions in a selected mass range against time or number of scans. TIC is a chromatogram for color-mass spectrometry.
Mass chromatograph
A plot of the ion intensities of a specified mass-to-charge ratio against time or scan number.
2D Data
In liquid-mass spectrometry, data containing only chromatograms, e.g., data collected by SIR or MRM (no mass spectrometry information).
3D Data
Data in liquid-liquid mass spectrometry that contains both chromatograms and mass spectra, such as data collected by FullScan (with mass spectrometry information).
Molecular Ion
An ion created by the loss of an electron from a molecule, with a mass-to-charge ratio equal to that of the molecule. The mass-to-charge ratio is equal to that of a molecule.
Quasi-molecular ion
An ion that is simply related to a molecule, and from which the molecular weight can be determined. For example, the ions formed by a molecule gaining or losing a hydrogen: (M+H) + , (M-H) - are the most common quasimolecular ions.
Fragment ions
Ion that is the product of the cleavage of a molecular ion
Parent and daughter ions
When any ion is further cleaved to produce an ion, the former is called the parent ion and the latter is called the daughter ion.
Singly Charged Ions and Multi-Charged Ions
Ions with only one charge are called singly charged ions, and ions with two or more charges are called multiply charged ions, and they often have non-integer charge ratios.
Isotope ions
Ions consisting of heavy isotopes of an element are called isotope ions, and always appear to the right of the corresponding molecular or fragment ions in the mass spectrum.
Nitrogen Rule
When a compound contains no nitrogen or an even number of nitrogen atoms, the molecular weight of the compound is even. When a compound contains an odd number of nitrogen atoms, the molecular weight of the compound is odd.
The molecular weight of a compound is even when the compound contains an odd number of nitrogen atoms and odd when the compound contains an odd number of nitrogen atoms.
Full Scan
An acquisition method that detects ions over a range of mass-to-charge ratios, and extracts one mass spectrum from each sampling point.
Scan Time
A parameter for Full Scan data acquisition, expressed in seconds, which indicates the time for the quadrupole to scan a certain range of charge-ratio ions.
Inter Scan Delay
This parameter is used to measure the interval between two scans in seconds.
Selected Ion Record(SIR)
Selects a peak in the mass spectrum that characterizes a substance for detection.
Dwell Time
A parameter in seconds that indicates the time for the ion to be released from the quadrupole when the data is collected by the SIR method.
MRM (Multiple Reaction Monitoring)
An acquisition method of tandem mass spectrometry that simultaneously detects parent ions and daughter ions in the SIR mode, characterized by a combination of high selectivity and high sensitivity, and suitable for quantitative analysis of trace target monitors.
Group 6 Concepts: Nomenclature and Terminology for Mass Spectrometers Ion source
The component of a mass spectrometer that ionizes the sample to produce ions. Examples: EI, FAB, ESI, APcI, etc.
Mass analyzer
A component of a mass spectrometer that separates ions according to their mass-to-charge ratio. Examples: quadrupole, ion trap, TOF, etc.
Ion detector
A component of a mass spectrometer that detects and amplifies the abundance of ions. Examples: photomultiplier, electron multiplier, multi-channel plate detector, etc.

Resolution (R).
The ability of an instrument to discriminate between two adjacent mass spectral peaks for a given sample.
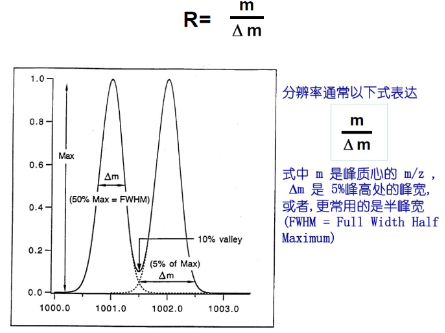
The smallest relative mass difference Δm that the instrument can resolve near the mass number m
At the same ion mass number, the higher the resolution, the smaller the
△m that can be resolved, and the higher the accuracy of the measured mass.
At the same resolution, the mass accuracy of measuring high mass number ions is low and the mass accuracy of measuring low mass number ions is high.
In other words, under the same requirement of mass accuracy, higher resolution is required to measure higher mass ions.
Sensitivity
Under the specified conditions, the selected compounds produced by a mass spectrometry peaks, the instrument produced by the unit sample response value.
Sensitivity is a mass spectrometry instrument to the sample volume sensing ability to assess the index. It is often expressed as a signal-to-noise ratio in experiments. In some types of mass spectrometers, sensitivity and resolution of inversely proportional,, improve the resolution at the same time, will reduce the sensitivity, and vice versa.
Absolute and relative sensitivity
Organic mass spectrometry is often used to measure the minimum detection of a standard sample sensitivity parameters. When the test conditions are the same, the smaller the sample size used, the higher the sensitivity of the instrument. The smaller the sample size used, the higher the sensitivity of the instrument, which is called the absolute sensitivity method.
Relative sensitivity method is a measure of the ability of the instrument to detect very small samples contained in other substances, usually ug / ml (ppm), ng / ml (ppb) that the relative sensitivity and the amount of injection is related to the increase in the amount of injection can increase the absolute amount of samples, so that the instrument is easier to detect the signal of the sample.
Signal to Noise Ratio
Signal to Noise Ratio (S/N= Signal to Noise Ratio): The ratio of the intensity of the spectral peak (signal) to the intensity of the noise.
Measurement of noise intensity
PtP(peak to peak): peak to peak noise value, select a section of the baseline noise maximum.
RMS (Root Mean Square): Root Mean Square noise value, the root mean square of the noise of a selected baseline segment.
Sensitivity must be measured under the same conditions (concentration, resolution, flow rate, injection volume, ions detected, etc.) in order to be comparable.
Mass range
The range between the lower and upper limits of ion mass that can be measured by a mass spectrometer. The unit of ion mass is the atomic mass unit (amu).
Mass discriminationeffects
The phenomenon in which some components of a mass spectrometer, such as the mass analyzer and ion detector, produce a biased response to ions of different masses.
Gas Chromatography and Mass Spectrometry (GC-MS)
An on-line coupling technique of a gas chromatograph and mass spectrometer for rapid separation and characterization of volatile mixtures.
Liquid Chromatography and Mass Spectrometry (LC-MS)
On-line coupling of high performance liquid chromatography (HPLC) and mass spectrometry (MS). Liquid chromatography acts as a special injector for mass spectrometry; in turn, mass spectrometry can be regarded as a detector for liquid chromatography.
Mass Spectrometry - Mass Spectrometry (MS-MS)
Refers to the on-line coupling of mass spectrometry and mass spectrometry, also known as tandem mass spectrometry, e.g., Q-Q (triple quadrupole tandem), Q-TOF.
Interface
A hardware device used to coordinate the output and input states of two instruments being used together.
Soft Ionization
A general term for low energy ionization methods such as chemical ionization and atmospheric pressure ionization.
Oops! Claude was unable to load the following URLs:
•https://mp.weixin.qq.com/s/cE4wDESH-tVPXgsd8OgABA